23 July 2022 | Statement | External URL: https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox
Reading time: 21 min (5670 words)
The WHO Director-General is hereby transmitting the Report of the second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox, held on Thursday, 21 July 2022, from 12:00 to 19:00 CEST.
The WHO Director-General is taking the opportunity to express his sincere gratitude to the Chairs and Members of the Committee, as well as to its Advisors, for their careful consideration of the issues regarding this outbreak, as well as for providing invaluable input for his consideration.
The Committee Members did not reach a consensus regarding their advice on determination of a Public Health Emergency of International Concern (PHEIC) for this event.
The WHO Director-General recognizes the complexities and uncertainties associated with this public health event.
Having considered the views of Committee Members and Advisors as well as other factors in line with the International Health Regulations, the Director-General has determined that the multi-country outbreak of monkeypox constitutes a Public Health Emergency of International Concern.
The WHO Director-General also considered the views of the Committee in issuing the set of Temporary Recommendations presented below.
===
Temporary Recommendations issued by the WHO Director-General in relation to the multi-country outbreak of monkeypox
These Temporary Recommendations apply to different groups of States Parties, based on their epidemiological situation, patterns of transmission and capacities. Each States Party, at any given point in time, falls either under Group 1 or under Group 2. Some State Parties may also fall under Group 3 and/or Group 4.
All Temporary Recommendations are expected to be implemented in full respect of established principles of human rights, inclusion and the dignity of all individuals and communities.
Group 1: States Parties, with no history of monkeypox in the human population or not having detected a case of monkeypox for over 21 days
1.a. Activate or establish health and multi-sectoral coordination mechanisms to strengthen all aspects of readiness for responding to monkeypox and stop human to human transmission.
1.b. Plan for, and/or implement, interventions to avoid the stigmatization and discrimination against any individual or population group that may be affected by monkeypox, with the goal of preventing further undetected transmission of monkeypox virus. The focus of these interventions should be: to promote voluntary self-reporting and care seeking behaviour; to facilitate timely access to quality clinical care; to protect the human rights, privacy and dignity of affected individuals and their contacts across all communities.
1.c. Establish and intensify epidemiological disease surveillance, including access to reliable, affordable and accurate diagnostic tests, for illness compatible with monkeypox as part of existing national surveillance systems. For disease surveillance purposes, case definitions for suspected, probable and confirmed cases of monkeypox should be adopted.
1.d. Intensify the detection capacity by raising awareness and training health workers, including those in primary care, genitourinary and sexual health clinics, urgent care / emergency departments, dental practices, dermatology, paediatrics, HIV services, infectious diseases, maternity services, obstetrics and gynaecology, and other acute care facilities.
1.e. Raise awareness about monkeypox virus transmission, related prevention and protective measures, and symptoms and signs of monkeypox among communities that are currently affected elsewhere in this multi-country outbreak (e.g., importantly, but not exclusively, gay, bisexual and other men who have sex with men (MSM) or individuals with multiple sexual partners) as well as among other population groups that may be at risk (e.g., sex workers, transgender people).
1.f. Engage key community-based groups, sexual health and civil society networks to increase the provision of reliable and factual information about monkeypox and its potential transmission to and within populations or communities that may be at increased risk of infection.
1.g. Focus risk communication and community support efforts on settings and venues where intimate encounters take place (e.g., gatherings focused on MSM, sex-on-premises venues). This includes engaging with and supporting the organizers of large and smaller scale events, as well as with owners and managers of sex on premises venues to promote personal protective measures and risk-reducing behaviour.
1.h. Immediately report to WHO, through channels established under the provision of the IHR, probable and confirmed cases of monkeypox, including using the minimum data set contained in the WHO Case Report Form (CRF).
1.i. Implement all actions necessary so as to be ready to apply or continue applying the set of Temporary Recommendations enumerated for Group 2 below in the event of first-time or renewed detection of one or more suspected, probable or confirmed cases of monkeypox.
Group 2: States Parties, with recently imported cases of monkeypox in the human population and/or otherwise experiencing human-to-human transmission of monkeypox virus, including in key population groups and communities at high risk of exposure
2.a. Implementing coordinated response
2.a.i. Implement response actions with the goal of stopping human-to-human transmission of monkeypox virus, with a priority focus on communities at high risk of exposure, which may differ according to context and include gay, bisexual and other men who have sex with men (MSM). Those actions include: targeted risk communication and community engagement, case detection, supported isolation of cases and treatment, contact tracing, and targeted immunization for persons at high risk of exposure for monkeypox.
2.a.ii. Empower affected communities and enable and support their leadership in devising, contributing actively to, and monitoring the response to the health risk they are confronting. Extend technical, financial and human resources to the extent possible and maintain mutual accountability on the actions of the affected communities.
2.a.iii. Implement response actions with the goal of protecting vulnerable groups (immunosuppressed individuals, children, pregnant women) who may be at risk of severe monkeypox disease. Those actions include: targeted risk communication and community engagement, case detection, supported isolation of cases and treatment, contact tracing. These may also include targeted immunization which takes into careful consideration the risks and benefits for the individual in a shared clinical decision-making.
2.b. Engaging and protecting communities
2.b.i. Raise awareness about monkeypox virus transmission, actions to reduce the risk of onward transmission to others and clinical presentation in communities affected by the outbreak, which may vary by context, and promote the uptake and appropriate use of prevention measures and adoption of informed risk mitigation measures. In different contexts this would include limiting skin to skin contact or other forms of close contact with others while symptomatic, may include promoting the reduction of the number of sexual partners where relevant including with respect to events with venues for sex on premises, use of personal protective measures and practices, including during, and related to, small or large gatherings of communities at high risk of exposure.
2.b.ii Engage with organizers of gatherings (large and small), including those likely to be conducive for encounters of intimate sexual nature or that may include venues for sex-on-premises, to promote personal protective measures and behaviours, encourage organizers to apply a risk-based approach to the holding of such events and discuss the possibility of postponing events for which risk measures cannot be put in place. All necessary information should be provided for risk communication on personal choices and for infection prevention and control including regular cleaning of event venues and premises.
2.b.iii. Develop and target risk communication and community engagement interventions, including on the basis of systematic social listening (e.g., through digital platforms) for emerging perceptions, concerns, and spreading of misinformation that might hamper response actions.
2.b.iv. Engage with representatives of affected communities, non-government organizations, elected officials and civil society, and behavioural scientists to advise on approaches and strategies to avoid the stigmatization of any individual or population groups in the implementation of appropriate interventions, so that care seeking behaviour, testing and access to preventive measures and clinical care is timely, and to prevent undetected transmission of monkeypox virus.
2.c. Surveillance and public health measures
2.c.i. Intensify surveillance for illness compatible with monkeypox as part of existing national surveillance schemes, including access to reliable, affordable and accurate diagnostic tests.
2.c.ii. Report to WHO, on a weekly basis and through channels established under the provision of the IHR, probable and confirmed cases of monkeypox, including using the minimum data set contained in the WHO Case Report Form (CRF).
2.c.iii. Strengthen laboratory capacity, and international specimens referral capacities as needed, for the diagnosis of monkeypox virus infection, and related surveillance, based on the use of nucleic acid amplification testing (NAAT), such as real time or conventional polymerase chain reaction (PCR).
2.c.iv. Strengthen genomic sequencing capacities, and international specimens referral capacities as needed, building on existing sequencing capacities worldwide, to determine circulating virus clades and their evolution, and share genetic sequence data through publicly accessible databases.
2.c.v. Isolate cases for the duration of the infectious period. Policies related to the isolation of cases should encompass health, psychological, material and essential support to adequate living. Any adjustment of isolation policies late in the isolation period would entails the mitigation of any residual public health risk.
2.c.vi. During the isolation period, cases should be advised on how to minimise the risk of onward transmission.
2.c.vii. Conduct contact tracing among individuals in contact with anyone who may be a suspected, probable, or confirmed case of monkeypox, including: contact identification (protected by confidentiality), management, and follow-up for 21 days through health monitoring which may be self-directed or supported by public health officers. Policies related to the management of contacts should encompass health, psychological, material and essential support to adequate living.
2.c.vii. Consider the targeted use of second- or third-generation smallpox or monkeypox vaccines (hereafter referred to as vaccine(s)) for post-exposure prophylaxis in contacts, including household, sexual and other contacts of community cases and health workers where there may have been a breach of personal protective equipment (PPE).
2.c.viv. Consider the targeted use of vaccines for pre-exposure prophylaxis in persons at risk of exposure; this may include health workers at high risk of exposure, laboratory personnel working with orthopoxviruses, clinical laboratory personnel performing diagnostic testing for monkeypox and communities at high risk of exposure or with high risk behaviours, such as persons who have multiple sexual partners.
2.c.x. Convene the National Immunization Technical Advisory Group (NITAG) for any decision about immunization policy and the use of vaccines. These should be informed by risks-benefits analysis. In all circumstances, vaccinees should be informed of the time required for protective immunity potentially offered by vaccination to be effective.
2.c.xi. Engage the communities at high risk of exposure in the decision-making process regarding any vaccine roll out vaccine.
2.d. Clinical management and infection prevention and control
2.d.i. Establish and use recommended clinical care pathways and protocols for the screening, triage, isolation, testing, and clinical assessment of suspected cases of persons with monkeypox; provide training to health care providers accordingly, and monitor the implementation of those protocols.
2.d.ii. Establish and implement protocols related to infection prevention and control (IPC) measures, encompassing engineering and administrative and the use of PPE; provide training to health care providers accordingly, and monitor the implementation of those protocols.
2.d.iii Provide health and laboratory workers with adequate PPE, as appropriate for health facility and laboratory settings, and provide all personnel with training in the use of PPE.
2.d.iv. Establish, update, and implement clinical care protocols for management of patients with uncomplicated monkeypox disease (e.g., keeping lesions clean, pain control, and maintaining adequate hydration and nutrition); with severe symptoms; acute complications; as well as for the monitoring and management of mid- or long-term sequelae.
2.d.v. Harmonise data collection and report clinical outcomes, using WHO Global Clinical Platform for monkeypox.
2.e. Medical countermeasures research
2.e.i. Make all efforts to use existing or new vaccines against monkeypox within a framework of collaborative clinical efficacy studies, using standardized design methods and data collection tools for clinical and outcome data, to rapidly increase evidence generation on efficacy and safety, collect data on effectiveness of vaccines (e.g., such as comparison of one or two dose vaccine regimens), and conduct vaccine effectiveness studies.
2.e.ii. Make all efforts to use existing or new therapeutics and antiviral agents for the treatment of monkeypox cases within a framework of collaborative clinical efficacy studies, using standardized design methods and data collection tools for clinical and outcome data, to rapidly increase evidence generation on efficacy and safety.
2.e.iii. When the use of vaccines and antivirals for monkeypox in the context of a collaborative research framework is not possible, use under expanded access protocols can be considered, such as the Monitored Emergency Use of Unregistered and Investigational Interventions (MEURI), under certain circumstances, using harmonized data collection for clinical outcomes (such as WHO Global Clinical Platform for Monkeypox).
2.f. International travel
2.f.i. Adopt and apply the following measures:
- Any individual:
- With signs and symptoms compatible with monkeypox virus infection; or being considered a suspect, probable, or confirmed case of monkeypox by jurisdictional health authorities; or
- Who has been identified as a contact of a monkeypox case and, therefore, is subject to health monitoring, should avoid undertaking any travel, including international, until they are determined as no longer constituting a public health risk. Exemptions include any individual who need to undertake travel to seek urgent medical care or flee from life-threatening situations, such as conflict or natural disasters; and contacts for whom pre-departure arrangements to ensure the continuity of health monitoring are agreed upon by sub-national health authorities concerned, or, in the case of international travel, by national health authorities;
- Cross-border workers, who are identified as contacts of a monkeypox case, and, hence, under health monitoring, can continue their routine daily activities provided that health monitoring is duly coordinated by the jurisdictional health authorities from both/all sides of the border.
2.f.ii. Establish operational channels between health authorities, transportation authorities, and conveyances and points of entry operators to:
- Facilitate international contact tracing in relation to individuals who have developed signs and symptoms compatible with monkeypox virus infection during travel or upon return;
- Provide communication materials at points of entry on signs and symptoms consistent with monkeypox; infection prevention and control; and on how to seek medical care at the place of destination;
WHO advises against any additional general or targeted international travel-related measures other than those specified in paragraphs 2.f.i and 2.f.ii.
Group 3: States Parties, with known or suspected zoonotic transmission of monkeypox, including those where zoonotic transmission of monkeypox is known to occur or has been reported in the past, those where presence of monkeypoxvirus has been documented in any animal species, and those where infection of animal species countries may be suspected including in newly affected countries
3.a. Establish or activate collaborative One Health coordination or other mechanisms at federal, national, subnational and/or local level, as relevant, between public health, veterinary, and wildlife authorities for understanding, monitoring and managing the risk of animal-to-human and human-to-animal transmission in natural habitats, forested and other wild or managed environments, wildlife reserves, domestic and peri-domestic settings, zoos, pet shops, animal shelters and any settings where animals may come into contact with domestic waste.
3.b. Undertake detailed case investigations and studies to characterize transmission patterns, including suspected or documented spillovers from, and spillback, to animals. In all settings, case investigation forms should be updated and adapted to elicit information on the full range of possible exposures and modes of both zoonotic and human-to-human transmission. Share the findings of these endeavours including ongoing case reporting with WHO.
Group 4: States Parties with manufacturing capacity for medical countermeasures
4.a. States Parties who have manufacturing capacity for smallpox and monkeypox diagnostics, vaccines or therapeutics should raise production and availability of medical countermeasures.
4.b. States Parties and manufacturers should work with WHO to ensure diagnostics, vaccines, therapeutics, and other necessary supplies are made available based on public health needs, solidarity and at reasonable cost to countries where they are most needed to support efforts to stop the onward spread of monkeypox.
Proceedings of the meeting
The second meeting of the IHR Emergency Committee on the multi-country outbreak of monkeypox was convened by Zoom, with the Chair and Vice-Chair being present in person in the premises of WHO headquarters, Geneva, Switzerland.
Members and Advisers joined by videoconference. Overall, 15 of the 16 Committee’s Members and all 10 Advisers to the Committee participated in the meeting.
The WHO Director-General welcomed the Committee, noting that he had reconvened them to assess the immediate and medium-term public health implications of the evolution of the multi-country monkeypox outbreak and provide their views on whether the event constitutes a public health emergency of international concern.
The WHO Director-General expressed concern about the number of cases, in an increasing number of countries, that have been reported to WHO and highlighted the challenges presented due to the complexity of transmission patterns in different Regions. He additionally stressed his awareness that determination of a Public Health Emergency of International Concern (PHEIC) involves the consideration of multiple factors, with the ultimate goal of protecting public health.
The Representative of the Office of Legal Counsel briefed the Members and Advisors on their roles and responsibilities and the mandate of the Emergency Committee under the relevant articles of the IHR.
The Ethics Officer from the Department of Compliance, Risk Management, and Ethics briefed Members and Advisers on their roles and responsibilities. Members and Advisers were also reminded of their duty of confidentiality as to the meeting discussions and the work of the Committee, as well as their individual responsibility to disclose to WHO, in a timely manner, any interests of a personal, professional, financial, intellectual or commercial nature that may give rise to a perceived or direct conflict of interest. Each Member and Adviser who was present was surveyed. No conflicts of interest were identified.
The meeting was handed over to the Chair of the Emergency Committee, Dr Jean-Marie Okwo-Bele who introduced the objectives of the meeting: to provide views to the WHO Director-General on whether the multi-country outbreak of monkeypox constitutes a PHEIC, and, if so, to review the proposed temporary recommendations to States Parties.
Presentations
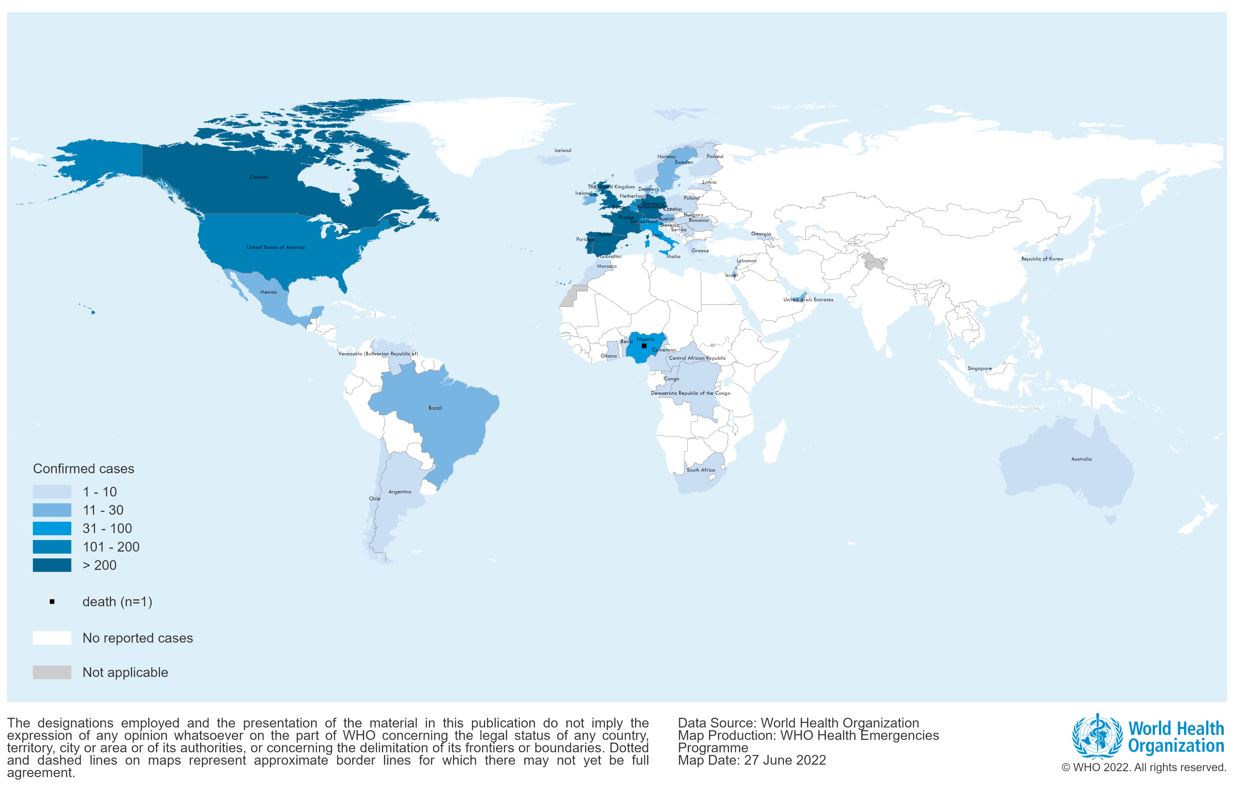
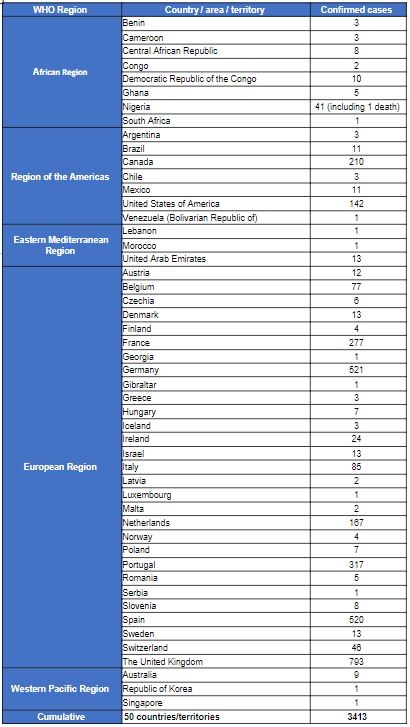
The WHO Secretariat presented the global epidemiological situation, highlighting that between 1 January 2022 and 20 July 2022, 14,533 probable and laboratory-confirmed cases (including 3 deaths in Nigeria and 2 in the Central African Republic) were reported to WHO from 72 countries across all six WHO Regions; up from 3,040 cases in 47 countries at the beginning of May 2022.
Transmission is occurring in many countries that had not previously reported cases of monkeypox, and the highest numbers of cases are currently reported from countries in the WHO European Region and the Region of the Americas.
The majority of reported cases of monkeypox currently are in males, and most of these cases occur among males who identified themselves as gay, bisexual and other men who have sex with men (MSM), in urban areas, and are clustered in social and sexual networks. Early reports of children affected include a few with no known epidemiological link to other cases.
There has also been a significant rise in the number of cases in countries in West and Central Africa, with an apparent difference in the demographic profile maintained than that observed in Europe and the Americas, with more women and children amongst the cases.
Mathematical models estimate the basic reproduction number (R0) to be above 1 in MSM populations, and below 1 in other settings. For example, in Spain, the estimated R0 is 1.8, in the United Kingdom 1.6, and in Portugal 1.4.
The clinical presentation of monkeypox occurring in outbreaks outside Africa is generally that of a self-limited disease, often atypical to cases described in previous outbreaks, with rash lesions localized to the genital, perineal/perianal or peri-oral area, that often do not spread further, and appears prior to the development of lymphadenopathy, fever, malaise, and pain associated with lesions.
The mean incubation period among cases reported is estimated at 7.6 to 9.2 days (based on surveillance data from the Netherlands, the United Kingdom of Great Britain and Northern Ireland (United Kingdom), and the United States of America (United States). The mean serial interval is estimated at 9.8 days (95% CI 5.9-21.4 day, based on 17 case-contact pairs in the United Kingdom).
A small number of cases have been reported among health workers. Investigations so far have not identified cases of occupational transmission, although investigations are ongoing.
The Secretariat noted that, although the number of cases and countries experiencing outbreaks of monkeypox appear to be rising, the WHO risk assessment has not changed since the first meeting of the Committee on 23 June 2022, and the risk is considered to be “moderate” at global level and in all six WHO Regions, except for European region, where it is considered to be “high”.
Modelling work conducted by European Centre for Disease Prevention and Control (ECDC) and the European Commission’s Health Emergency Preparedness and Response Authority (HERA) suggests that isolation of cases and contact tracing could be effective in bringing the outbreak under control. However, the operational experience gained to date in responding to this event, indicates that the implementation of such interventions in practice is extremely challenging – the identification of cases is hampered by barriers to access diagnostic testing; the isolation of cases for 21 days is difficult in the current COVID-19 pandemic-related post-lockdowns context; and contact tracing is difficult as contacts are often multiple and may be anonymous. The modelling by ECDC and HERA is suggesting that the addition of vaccination-related interventions can increase the chances of controlling the outbreak, with pre-exposure prophylaxis of individuals at high-risk of exposure appearing to be the most effective strategy to use vaccines when contact tracing is less effective, or impracticable. However, the limited data on vaccine effectiveness against monkeypox constitutes one of the limitations of the modelling work conducted. Additionally, the operationalization of such vaccination strategy presents challenges, including those related to vaccine access.
The genome sequence of the virus obtained in several countries shows some divergence from the West African clade. Work is ongoing to understand whether the observed genomic changes lead to phenotypic changes such as enhanced transmissibility, virulence, immune escape, resistance to antivirals, or reduced impact of countermeasures.
Although many species of animals are known to be susceptible to the monkeypox virus in the natural setting (e.g., rope squirrels, tree squirrels, Gambian pouched rats, dormice, non-human primates), there is the potential for spillback of the virus from humans to other susceptible animal species in different settings. To date, there is currently no documented evidence of instances of anthropozoonotic transmission available to the WHO Secretariat or its One Health partners the Food and Agriculture Organization (FAO) and the World Organisation for Animal Health (WOAH).
The WHO Secretariat also outlined the WHO response so far, and the ongoing work to develop the WHO Strategic Readiness and Response Plan for monkeypox, being its overall goal to stop human-to-human transmission.
Representatives of Spain, the United Kingdom, the United States, Canada and Nigeria updated the Committee (in this order) on the epidemiological situation in their countries and their current response efforts. With the exception of Nigeria, the remaining four countries reported that 99% of cases were occurring in MSM, and mainly among those with multiple partners.
In Spain, cases have been decreasing over the past few weeks, but it is likely the data are incomplete because of delays in reporting. Most cases have been reported in major urban areas, with very few reports of cases among females and children who had epidemiological links to MSM. Pre-exposure prophylaxis with vaccination is being offered to health workers, contacts and people living with HIV, but vaccine supplies are low.
The United Kingdom reported on a few severe cases of monkeypox (including encephalitis), and it is also planning to modify its case definition for monkeypox, to include newly recognized conditions such as proctitis. Environmental investigations have identified monkeypox virus DNA (presumed to be infectious because of moderate Ct values) on surfaces in hospitals and households. The vaccine strategy is targeted and aims to interrupt transmission through post-exposure prophylaxis and pre-exposure prophylaxis among MSM at highest risk.
In the United States, cases of monkeypox are widely distributed across the country, although most cases are concentrated in three large cities. While a few cases have occurred in children and a pregnant woman, 99% are related to male-to-male sexual contact.
In Canada, 99% of cases have occurred among MSM, and the country is taking a broad approach to pre-exposure prophylaxis, given the challenges with contact tracing; and is strongly focused on engagement with community-led organizations supporting key affected populations groups.
Nigeria recorded a little over 800 cases of monkeypox between September 2017 and 10 July 2022 and has seen at 3% case fatality ratio among confirmed cases. Cases are predominantly in men aged 31 to 40 years; there was no evidence of sexual transmission presented. The highest number of annually reported cases since 2017 has been observed in 2022.
Following the presentations, the Committee Members and Advisers proceeded with a questions and answers session for both the Secretariat and the presenting countries.
The Committee continues to be concerned about a broad range of issues, including the following: the need for further understanding of transmission dynamics; the impact of the fear of stigma on health-seeking behaviour among MSM; the potential implications on rights-based delivery of care by Ministries of Health and other authorities; the challenges related to the use of public health and social measures to stop onward transmission, including isolation, access to testing and contact tracing, particularly because of multiple anonymous contacts; planned large local and international gatherings focused on MSM and associated public and private satellite events, conducive for increased opportunities for exposure through intimate sexual encounters and subsequent amplification of the outbreak; the need for continuous evaluation of interventions may have have had an impact on transmission (e.g., one-dose versus two-dose vaccination regimens and vaccine effectiveness in general, given the apparent permucosal exposures that are causing infection in some cases); and the identification of key activities for targeted risk communications and community engagement, working in close partnership with affected communities, and providing the necessary support for community-led organizations to play their important role in the response to the outbreak.
There was particular concern about how vaccines and antivirals would be priced and distributed in the near future and made available in an equitable manner.
Deliberative session
The Committee reconvened in a closed meeting to examine the questions in relation to whether the event constitutes a PHEIC or not, and if so, to consider the Temporary Recommendations, drafted by the WHO Secretariat in accordance with IHR provisions.
At the request of the Chair, the WHO Secretariat reminded the Committee Members of their mandate and recalled the definition of a PHEIC under the IHR: an extraordinary event, which constitutes a public health risk to other States through international spread, and which potentially requires a coordinated international response.
The Committee reviewed evidence gathered by the Secretariat against the considerations proposed during its first meeting for re-assessing the outbreak. The Committee noted the generally moderate level of confidence in the available data to make any informed determination on these considerations.
Of the nine considerations put forward, based on currently available data, two of them have seen a significant change since the previous meeting – an increase number of countries reporting the first case(s) of monkeypox, and an increase of the number of cases in some West and Central African countries. There was evidence of a small increase of the overall growth rate associated with the outbreak. While cases among health workers have been reported, most reported community exposure. A limited number of cases among sex workers has been reported from case reports and social media listening. Secondary transmission to some children and women was reported. Limited transmission was reported to have been observed among vulnerable groups (immunosuppressed individuals, pregnant women, or children, although a small number of children were reported not to have an epidemiological link to another case. While cases experiencing severe pain continue to be reported, with some hospitalizations required to manage pain or secondary infection, and while clinical severity of cases overall remained generally unchanged since the previous meeting, a few severe cases, two ICU admissions and five deaths have been reported. At the present time, there is no data currently available about potential spillback from humans to animals. With regards to the potential changes in the virus genome, investigations are ongoing in relation to the reports of changes that may affect features of the virus. There has to date not been any reported circulation of the virus clade normally present in Central Africa outside of the usual settings.
Conclusions
Committee Members expressed a range of views on the considerations before them. They were unable to reach consensus regarding advice to the WHO Director-General on whether the multi-country outbreak of monkeypox should or should not be determined to constitute a Public Health Emergency of International Concern (PHEIC). Supportive elements regarding the views expressed by the Members of the Committee in favour or not in favour of such a determination are summarized below. Such views reflected:
Committee Members’ views in support of the prospective determination of a PHEIC
- The multi-country outbreak of monkeypox meets all the three criteria defining a PHEIC contained in Article 1 of the Regulations (1. an extraordinary event […] 2. constitut[ing] a public health risk to other States through the international spread of disease 3. which may potentially require a coordinated international response);
- The moral duty to deploy all means and tools available to respond to the event, as highlighted by leaders of the LGBTI+ communities from several countries, bearing in mind that the community currently most affected outside Africa is the same initially reported to be affected in the early stages of HIV/AIDS pandemic;
- The observed rising trends in the number of cases reported globally, in an increasing number of countries, and, yet, likely to reflect an underestimation of the actual magnitude of the outbreak(s);
- The cases of monkeypox reported in children and pregnant women, which are reminiscent of the initial phases of the HIV pandemic;
- Future waves of monkeypox cases are expected as the monkeypox virus is introduced in additional susceptible populations;
- The modes of transmission sustaining the current outbreak are not fully understood;
- The changes in the clinical presentation of cases of monkeypox currently observed with respect to the clinical picture known to date;
- The need to generate further evidence related to the effectiveness of the use of both, pharmaceutical and non-pharmaceutical measures in controlling the outbreak;
- The significant morbidity associated with the monkeypox outbreak(s);
- The potential future implications on public health and health services if the disease were to establish itself in the human population across the world, particularly for an orthopoxvirus causing human disease, as global immunity has greatly declined after smallpox was eradicated;
The perceived benefits associated with the prospective determination of a PHEIC include:
- Maintaining a heightened level of awareness and alert, which would increase the probability of stopping human-to-human transmission of monkeypox virus;
- Boosting political commitment towards response efforts;Increasing opportunities for funds to be released for response, and research purposes, as well as for the mitigation of the socioeconomic impact of the disease;
- Boosting international coordination of response efforts, in particular to secure equitable access to vaccines and antivirals;
- The possible stigmatization, marginalization, and discrimination that may result from the prospective determination of a PHEIC should not be regarded as deterrent to do so, and would need to be addressed.
Committee Members’ views NOT in support of the prospective determination of a PHEIC
- The overall global risk assessment presented by the WHO Secretariat remained unchanged with respect to that presented to the Committee on 23 June 2022;
- The greatest burden of the outbreak is currently reported in 12 countries in Europe and in the Americas, with no indications, based on currently available data, of an exponential increase in the number of cases in any of those countries, and early signs of stabilization or declining trends observed in some countries;
- The vast majority of cases are observed among MSM with multiple partners, and, despite the operational challenges, there is the opportunity to stop ongoing transmission with interventions targeted to this segment of the population. Cases observed beyond this population group, including among health workers are, to date, limited;
- The severity of the disease is perceived to be low;
- The epidemic is gaining maturity, with future waves expected, and clearer indications about the effectiveness of policies and interventions are being generated;
The potential risks of hampering response efforts through the prospective determination of a PHEIC are perceived as outweighing the benefits of the latter for the following reasons:
- The stigma, marginalization, and discrimination that a determination of a PHEIC may generate against the currently affected communities, especially in countries where homosexuality is criminalized, LGBTI+ communities are not well established and engaged in a dialogue with governments. Communities in some countries have reportedly indicated that minimizing stigma associated with monkeypox – which unlike HIV infection may be a visible condition– requires developing novel approaches, which could be challenging in the context of a PHEIC;
- Action taken by the WHO Secretariat since May 2022 to raise the alert in relation to the unfolding monkeypox outbreak, including convening the Committee, appear to be effective, in triggering immediate response efforts in many countries in the northern hemisphere;
- Technical guidance issued by the Secretariat to inform national response efforts is regarded as adequate and comprehensive, with no identified impediments preventing its implementation worldwide;
- For West and Central African countries, where capacity building for surveillance, laboratory, and response is needed, the determination of a PHEIC may not be regarded as a tool for triggering nor for boosting such efforts;
- The determination of a PHEIC would unnecessarily and artificially increase the perception of the risk of the disease in the general public, which, in its turn, would translate into generating demand for vaccines, which should be used wisely;
Not determining a PHEIC would not mean “business as usual”. The communication of the WHO Director-General decision would still be an opportunity to convey the needed continuity of the full range of necessary public health actions, beyond a mere high visibility determination.
Following the deliberations, Committee Members provided input to the proposed Temporary Recommendations previously outlined, should the WHO Director-General determine that the Multi-country outbreak of monkeypox constitutes a PHEIC.








You must be logged in to post a comment.